Introduction
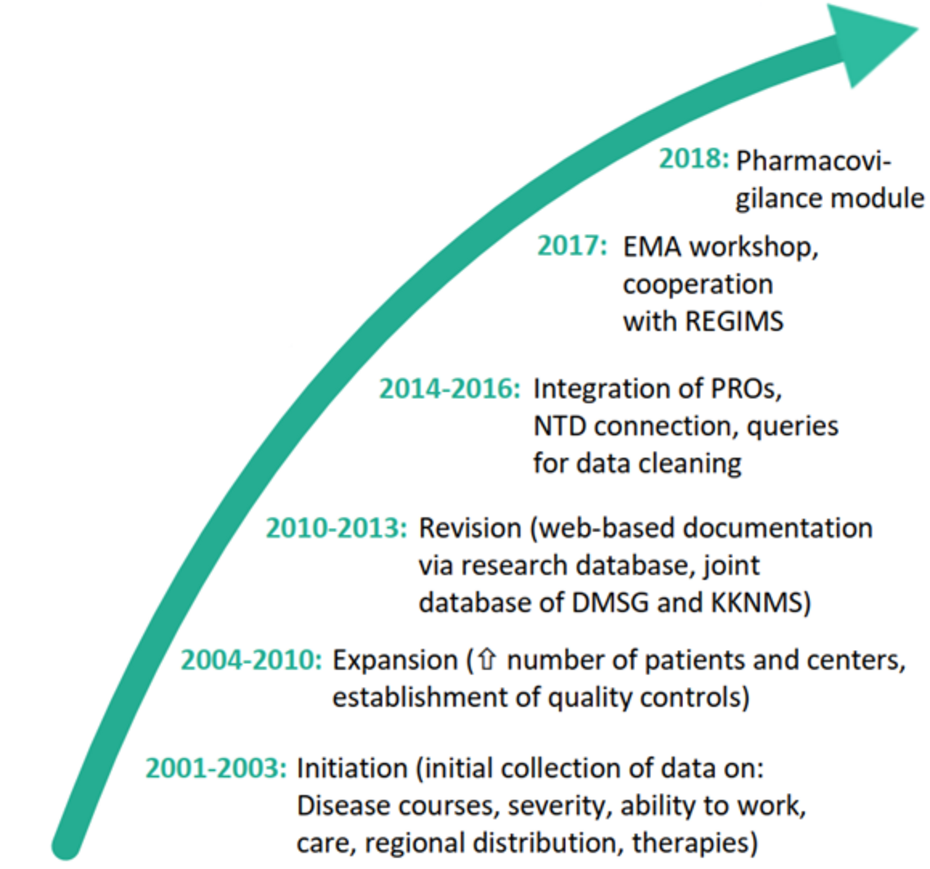
In 2001, the German Multiple Sclerosis Society (DMSG) initiated the installation of a Multiple Sclerosis Registry (MS Registry) for Germany. For this purpose, the MS Research and Project Development gGmbH (MSFP) was founded to manage the MS Registry. In 2005, the MS Registry started regular operations and has been continually developed and expanded since then.
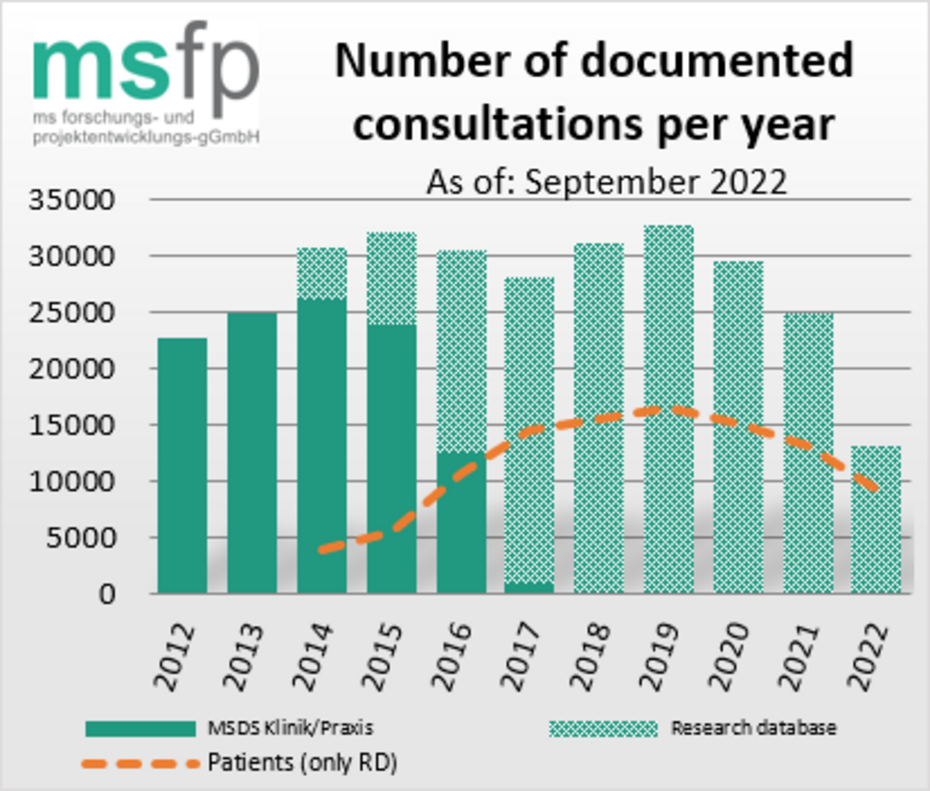
Most recently, almost 13,000 patients per year were reported by the centers listed by the DMSG. On average, two consultations per patient and year were documented.
Awards for (rehab) clinics and practices based on the guidelines of the DMSG, Bundesverband e.V.
Participation in the MS Registry of the DMSG, Bundesverband e. V. is a prerequisite for receiving the certificate "MS Center", "Specialized MS Center", and "MS Rehabilitation Center", awarded by the DMSG. The certificates are awarded to university clinics, acute care clinics, rehabilitation clinics, MS outpatient clinics, and neurological practices if they meet the specified criteria. Adherence must be confirmed every two years. The criteria catalog developed by independent MS experts focuses on a guideline-based treatment by neurologists and professionals specialized in MS, as well as on disabled accessible equipment of the facility. Depending on the center type, a minimum number of MS patients must be treated per year in the centers.
Part of these treated MS patients must be re-corded in the MS Registry. MS Special Centers thus must document at least 150 data sets per year, MS Rehabilitation Centers at least 80 or 120, and MS Centers at least 80. At the moment, the DMSG has decorated 70 centers as "Specialized MS Center", 94 as "MS Center", and 19 as "MS Rehabilitation Center". The geographic distribution of the centers in Germany is mostly homogen, with a slight West-East and South-North gradient as well as clusters in metropolitan areas. You can find the exact distribution and number of centers here.
MS Registry documentation
Since 2014, there has been a web-based, platform- and device-independent research database for the documentation of MS Registry data. The research database relies on established tools and the concepts of the TMF e. V. for collaborative research. It is also possible to integrate so-called patient-reported outcomes (PRO), for instance for quality of life data that are self-documented by the patients via app or web.
International cooperation
The MS Registry participates in the EMSP-Initiative "Multiple Sclerosis Data Alliance" (MSDA), whose main purpose is to implement a minimal data set and quality standards, approved by the European Medicine Agency (EMA), in as many MS data registries and cohorts as possible in order to enable the use of registries for questions of safety and efficiency of MS therapies in the future. Since the beginning of the Corona pandemic, the MS Registry has participated in the Global Data Sharing Initiative of MSDA and the MS International Federation on COVID-19. The latest analyses conclude that the use of anti-CD20 agents (both ocrelizumab and rituximab), as well as male gender, older age, progressive MS and higher disability are associated with more severe course of COVID-19. The results were recently published in Neurology as "Updated Results of the COVID-19 in MS Global Data Sharing Initiative".
Supporters of the MS Registry
The MS Registry of the DMSG has been financed since 2001 by the DMS Foundation and the DMSG, Bundesverband e. V. The MSFP receives project funding from the Innovation Fund of the G-BA and the German Pension Insurance (DRV Bund), among others. Since 2018, companies from the pharmaceutical industry have also been supporting the MS Registry as part of a multistakeholder funding. This primarily supports the establishment and operation of the recording of adverse events.
In 2018, the MSFP received financial support from Novartis and Merck Serono, which was used, among other things, to implement the EMA recommendations of a harmonized registry dataset. In 2019, Biogen and Celgene (BristolMeyerSquibb) joined the multistakeholder sponsorship. In 2020, the registry operations, particularly in the area of drug safety and the payment of documentation fees to participating centers, was supported by Biogen, Bristol Myers Squibb, Merck, Roche, and Sanofi with unified contributions. In 2021 and 2022, Biogen, Bristol Myers Squibb, Merck, Novartis, Roche and Sanofi participated with uniform contributions. The 2023 operation of the MS-Registry was supported by Biogen, Bristol Myers Squibb, Merck, Novartis Pharma and Roche. In 2024 Bristol Myers Squibb, Merck, Novartis Pharma and Roche continue to support the Registry and are joined by TG Therapeutics.


![[Translate to English:] DMS Logo](/fileadmin/_processed_/3/d/csm_dms_stiftung_0542dfbcb6.jpg)
![[Translate to English:] DMSG Aktionslogo](/fileadmin/_processed_/2/7/csm_dmsg_e5faf789ca.png)



